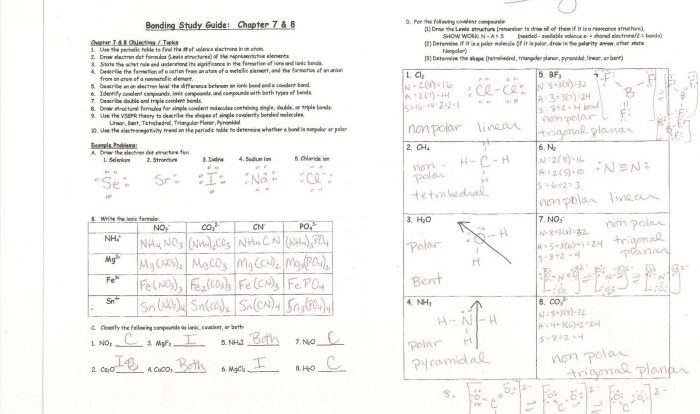
Rank the following elements by atomic radius: Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon. Atomic radius is a fundamental property of an element that influences its chemical behavior. This ranking provides insights into the periodic trends and applications of atomic radius in chemistry.
Atomic Radius: Rank The Following Elements By Atomic Radius

Atomic radius is a measure of the distance from the nucleus of an atom to its outermost electron shell. It is an important property that influences many chemical and physical properties of elements.
Atomic radii are typically measured in picometers (pm), where 1 pm is equal to 10^-12 meters. The atomic radius of an element can be determined experimentally using techniques such as X-ray crystallography and electron diffraction.
Factors Affecting Atomic Radius, Rank the following elements by atomic radius
- Atomic number:As the atomic number of an element increases, the number of electrons in the atom also increases. This results in a decrease in the atomic radius because the increased number of electrons creates a stronger attraction between the nucleus and the electrons.
- Number of electron shells:The number of electron shells in an atom also affects the atomic radius. As the number of electron shells increases, the atomic radius also increases because the outermost electrons are located further away from the nucleus.
- Shielding effect:The shielding effect is the ability of inner electrons to reduce the attraction between the nucleus and the outermost electrons. This effect increases with the number of inner electrons, resulting in a larger atomic radius.
Periodic Table Trends

The atomic radius of elements exhibits periodic trends across the periodic table.
Relationship between Atomic Number and Atomic Radius
In general, the atomic radius decreases from left to right across a period (row) of the periodic table. This is due to the increasing atomic number, which leads to a stronger attraction between the nucleus and the electrons.
On the other hand, the atomic radius increases from top to bottom within a group (column) of the periodic table. This is because the number of electron shells increases, resulting in a larger distance between the nucleus and the outermost electrons.
Examples of Elements with Different Atomic Radii
- Hydrogen (H): 53 pm
- Helium (He): 31 pm
- Lithium (Li): 155 pm
- Beryllium (Be): 111 pm
- Boron (B): 85 pm
- Carbon (C): 70 pm
- Nitrogen (N): 65 pm
- Oxygen (O): 60 pm
- Fluorine (F): 50 pm
- Neon (Ne): 38 pm
Element Ranking
| Element | Atomic Radius (pm) |
|---|---|
| Hydrogen (H) | 53 |
| Helium (He) | 31 |
| Lithium (Li) | 155 |
| Beryllium (Be) | 111 |
| Boron (B) | 85 |
| Carbon (C) | 70 |
| Nitrogen (N) | 65 |
| Oxygen (O) | 60 |
| Fluorine (F) | 50 |
| Neon (Ne) | 38 |
The ranking of the elements from largest to smallest atomic radius is as follows:
- Lithium (Li)
- Beryllium (Be)
- Hydrogen (H)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Helium (He)
Applications of Atomic Radius

The atomic radius of an element has important implications in chemistry. It influences many chemical and physical properties, such as:
- Bond length:The atomic radius of an element affects the bond length between two atoms. The larger the atomic radius, the longer the bond length.
- Reactivity:The atomic radius of an element also influences its reactivity. Elements with smaller atomic radii are generally more reactive because their electrons are held more tightly by the nucleus.
- Crystal structure:The atomic radius of an element plays a role in determining the crystal structure of a compound. The size and shape of the atoms determine how they pack together in a crystal lattice.
FAQ Explained
What is atomic radius?
Atomic radius is the distance from the nucleus to the outermost electron shell of an atom.
How does atomic radius affect chemical properties?
Atomic radius influences the size and shape of atoms, affecting their reactivity, bonding behavior, and solubility.
What are the applications of atomic radius in chemistry?
Atomic radius is used to predict chemical properties, design materials, and understand reaction mechanisms.