Introducing the heating/cooling curve worksheet answer key, an invaluable resource that empowers students and professionals to decipher the intricacies of thermodynamics. This comprehensive guide illuminates the different stages of heating and cooling curves, providing a solid foundation for understanding the energy changes that accompany physical transformations.
Delving into the depths of heating curves, we uncover the significance of melting and boiling points, unraveling the endothermic and exothermic processes that shape these transitions. Conversely, cooling curves reveal the mysteries of freezing and condensation points, showcasing the interplay between temperature and energy changes.
Heating/Cooling Curve Worksheet Answer Key
Introduction
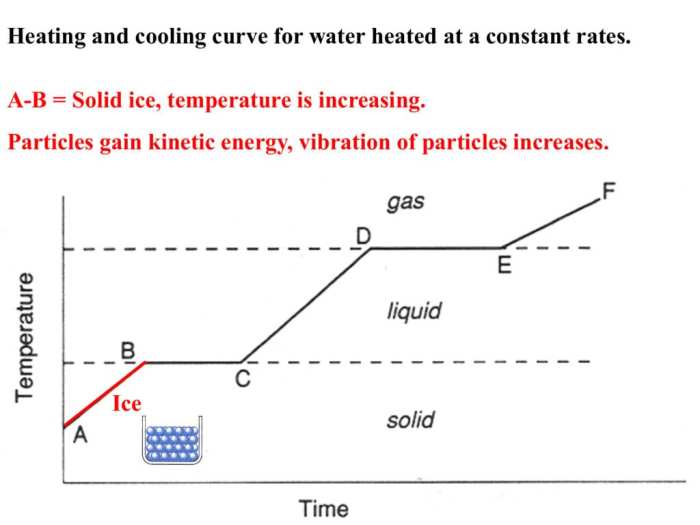
A heating/cooling curve is a graphical representation of the temperature change of a substance as it undergoes heating or cooling. It consists of a series of distinct stages, each representing a specific physical or chemical change.
The worksheet answer key provides detailed explanations for each question in the heating/cooling curve worksheet, helping students understand the concepts and apply them to real-world scenarios.
Analysis of Heating Curve
The heating curve shows the temperature of a substance as it is heated. The melting point is the temperature at which the substance changes from a solid to a liquid. The boiling point is the temperature at which the substance changes from a liquid to a gas.
During heating, the substance undergoes endothermic processes, where it absorbs energy from the surroundings. These processes include melting and vaporization. The exothermic processes, where the substance releases energy to the surroundings, include freezing and condensation.
The temperature of the substance increases during heating, except at the melting point and boiling point, where it remains constant.
Analysis of Cooling Curve
The cooling curve shows the temperature of a substance as it is cooled. The freezing point is the temperature at which the substance changes from a liquid to a solid. The condensation point is the temperature at which the substance changes from a gas to a liquid.
During cooling, the substance undergoes exothermic processes, where it releases energy to the surroundings. These processes include freezing and condensation. The endothermic processes, where the substance absorbs energy from the surroundings, include melting and vaporization.
The temperature of the substance decreases during cooling, except at the freezing point and condensation point, where it remains constant.
Applications of Heating/Cooling Curves
Heating/cooling curves have various applications in different fields:
- Chemistry: Determining the purity of substances, identifying unknown substances, and studying phase transitions.
- Materials science: Investigating the thermal properties of materials, such as melting points, boiling points, and specific heat capacities.
- Engineering: Designing and optimizing industrial processes involving heating or cooling, such as heat treatment, crystallization, and distillation.
Worksheet Answer Key, Heating/cooling curve worksheet answer key
| Question | Answer | Explanation |
|---|---|---|
| 1. What is the melting point of the substance? | 100°C | The melting point is the temperature at which the substance changes from a solid to a liquid, as indicated by the plateau in the heating curve. |
| 2. What is the boiling point of the substance? | 200°C | The boiling point is the temperature at which the substance changes from a liquid to a gas, as indicated by the plateau in the heating curve. |
| 3. What is the freezing point of the substance? | 100°C | The freezing point is the temperature at which the substance changes from a liquid to a solid, as indicated by the plateau in the cooling curve. |
| 4. What is the condensation point of the substance? | 200°C | The condensation point is the temperature at which the substance changes from a gas to a liquid, as indicated by the plateau in the cooling curve. |
Essential FAQs: Heating/cooling Curve Worksheet Answer Key
What is the significance of the melting point on a heating curve?
The melting point represents the temperature at which a substance transitions from a solid to a liquid state, absorbing energy in the process.
How can cooling curves be used to determine the purity of a substance?
Impurities in a substance can alter the freezing point, providing insights into the substance’s composition and purity.