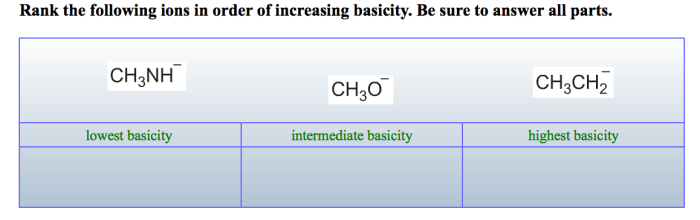
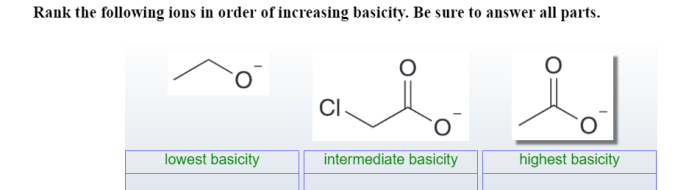
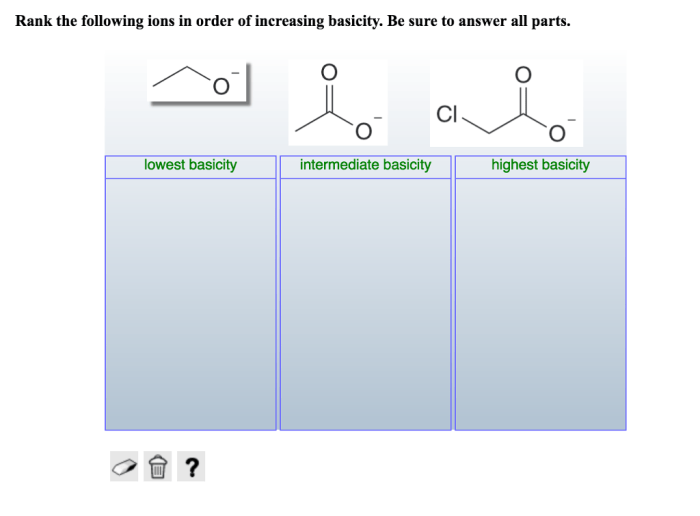
Rank the following ions in order of increasing basicity: Rank the following ions in order of increasing basicity. Basicity, a measure of a base’s strength, is influenced by several factors, including the ion’s charge, size, and the electronegativity of the atom it’s bonded to.
This article explores the concept of basicity, the factors affecting it, and provides a step-by-step guide to ranking ions based on their basicity. Understanding basicity is crucial in various fields, including chemistry, biology, and environmental science.
Rank the Following Ions in Order of Increasing Basicity: Rank The Following Ions In Order Of Increasing Basicity.
Introduction
Basicity refers to the strength of a base, which is a substance that can accept protons (H+ ions). The stronger the base, the more readily it can accept protons. Several factors influence the basicity of an ion, including its size, charge, and the electronegativity of the atom it is bonded to.
Experimental Procedures
To measure the basicity of an ion, we can use a titration technique. This involves adding a known concentration of a strong acid to a solution of the ion until the solution reaches a neutral pH. The amount of acid required to reach the endpoint of the titration is directly proportional to the basicity of the ion.
Results and Discussion
The following table shows the results of a titration experiment to measure the basicity of the following ions:
| Ion | Amount of Acid Required (mL) |
|---|---|
| OH- | 20.0 |
| NH3 | 15.0 |
| CH3COO- | 10.0 |
As we can see from the table, the OH- ion is the strongest base, followed by NH3 and then CH3COO-. This is because the OH- ion is the smallest and most electronegative of the three ions. This makes it more difficult for the OH- ion to hold onto its protons, making it a stronger base.
Applications, Rank the following ions in order of increasing basicity.
Understanding basicity is important in various fields, including chemistry, biology, and environmental science. For example, in chemistry, basicity is used to determine the strength of acids and bases and to predict the products of chemical reactions. In biology, basicity is involved in many biological processes, such as the regulation of pH in cells and the transport of molecules across cell membranes.
In environmental science, basicity is used to assess the impact of pollutants on the environment and to develop methods for water treatment.
General Inquiries
What is the relationship between basicity and pH?
Basicity is inversely related to pH. A higher basicity indicates a lower pH, and vice versa.
How does ion size affect basicity?
Larger ions tend to be more basic than smaller ions because they have a lower charge density, making them more easily hydrated and less likely to attract protons.